The symbol for the unit mole is mol. One mole of a substance contains the same number of the stated particles, atoms, molecules, or ions as one mole of any other substance. The number of atoms, molecules or ions in a mole (1 mol) of a given substance is the Avogadro constant. The value of the Avogadro constant is 6.02 x 10 23 per mole.. GCSE; Edexcel; Mole calculations (higher) - Edexcel The mole - Higher. The mole is the unit for the amount of substance. The number of particles in a substance can be found using the Avogadro.

Everything You Need To Know About Dr. Does Chemistry Test Answer Key » Answer Key Hub Your
GCSE Chemistry Moles Revision Webclass YouTube

Moles Exam Question 91 GCSE Chemistry OCR, AQA, Edexcel YouTube

Moles Worksheet Answers Wowway Biz

How To Achieve Grade 9 In GCSE Chemistry The Exam Coach

AQA GCSE Chemistry (Science) Calculating Moles Lesson Teaching Resources Chemistry, Gcse
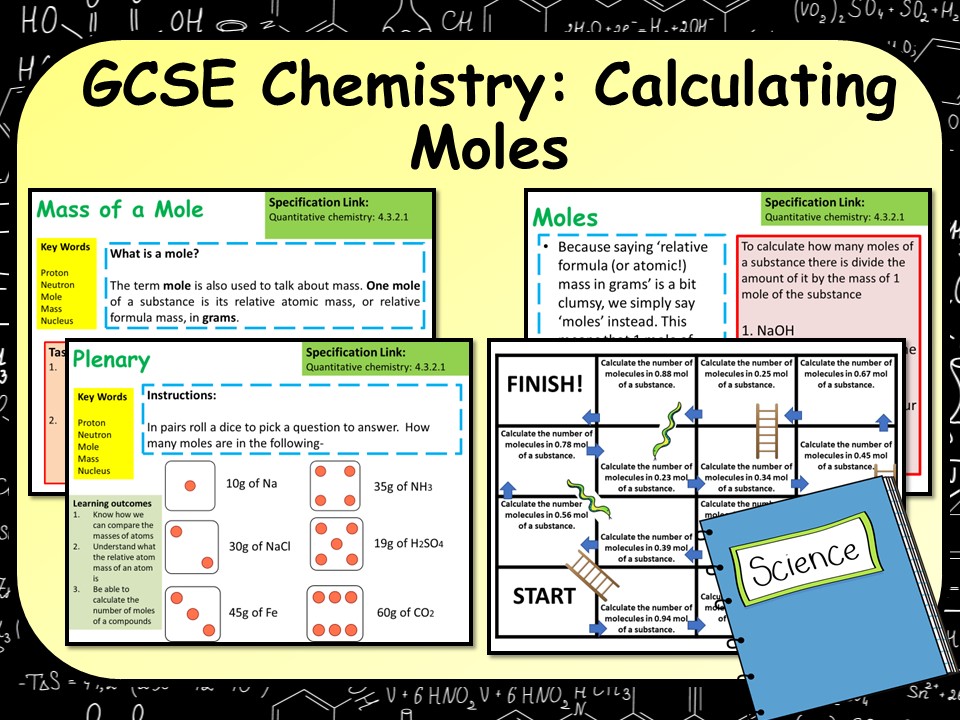
AQA GCSE Chemistry (Science) Calculating Moles Lesson Teaching Resources

GCSE Chemistry Moles and Equations (higher tier) Teaching Resources
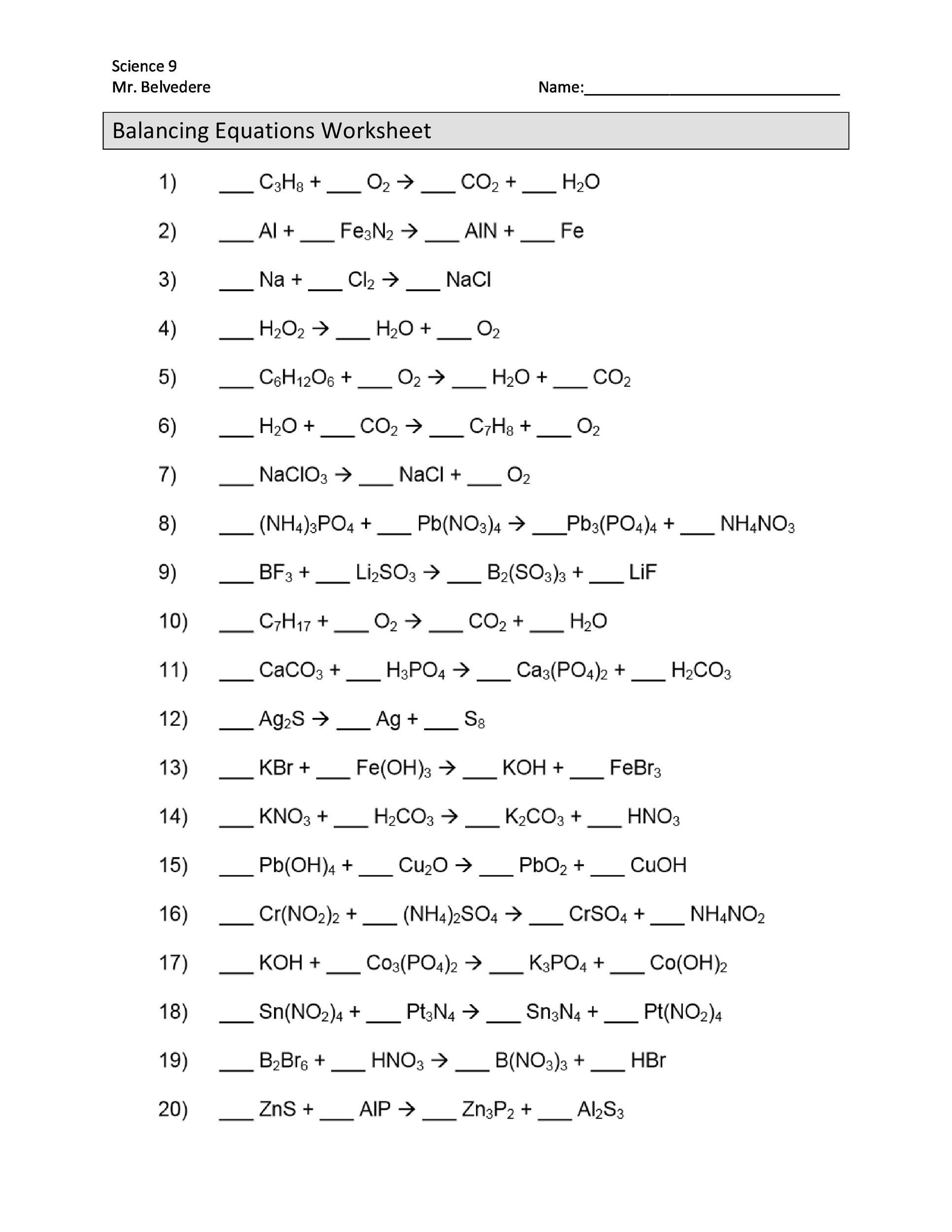
Balancing Equations Worksheet 8th Grade

GCSE Chemistry Revision "Calculating Moles of an Element" YouTube

GCSE Chemistry Moles 3 Number of Particles YouTube

Mole Worksheet 1 Answer Key
Moles Exam Questions The Student Room

AQA GCSE Chemistry (Science) Calculating Moles Lesson Teaching Resources Gcse chemistry, Aqa

Mole Concept All Numerical Clear Cut Pdf,Photos, Full Solved Practice Questions

Secondary 3 Chemistry Worksheet (Mole Concept)
GCSE Chemistry Moles
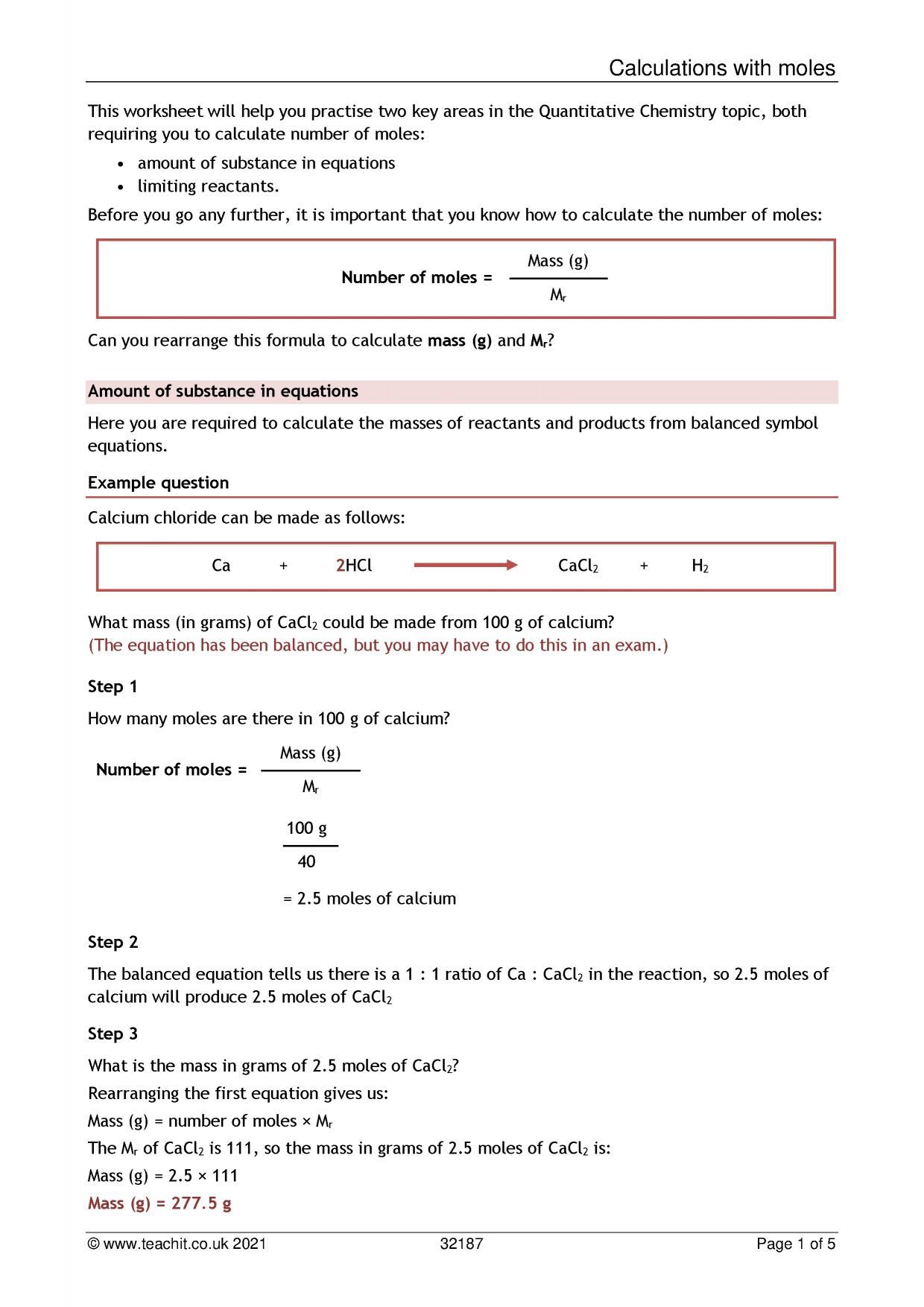
Using moles to calculate amount of substance/limiting reactants GCSE Science Teachit Science

GCSE Science GCSE Chemistry Moles Questions PDF Mole (Unit) Chemistry

Aqa Paper 1 Question 5 Past Papers Aqa Chemistry Paper 1 214 Quick Hot Sex Picture
The mole is one of the most, if not the most, important quantity in chemistry. One mole is equal to 6.022 \times 10^{23} of whatever it is that is being measured.To give a sense of just how massive a number this is, if you were to line up one mole of world cup standard footballs, the line would be around a thousand times longer than the width of the milky way.. The symbol for the unit mole is mol. One mole of a substance contains the same number of the stated particles, atoms, molecules, or ions as one mole of any other substance. The number of atoms, molecules or ions in a mole (1 mol) of a given substance is the Avogadro constant. The value of the Avogadro constant is 6.02 x 1023 per mole.