Electrochemistry involves the interconversion of electrical and chemical energy. The available energy used up in the chemical reaction is Gibbs free energy. In a chemical reaction, the Gibbs free energy is equal to the difference of enthalpy with the product of temperature and entropy. The change in Gibbs free energy is positive, negative, or zero.. Gibbs Free Energy relates enthalpy and entropy to determine whether a reaction will proceed spontaneously at constant temperature and pressure. To calculate it you can use ΔG° = ΔH° - TΔS for reactions under standard conditions.. Electrochemistry Balancing Redox Reactions Galvanic/Voltaic Cells, Calculating Standard Cell Potentials.

Gibbs Free Energy Change Problem 1 Electrochemistry Chemistry Class 12 YouTube

Gibbs Free Energy Formula In Electrochemistry Mrschimomot
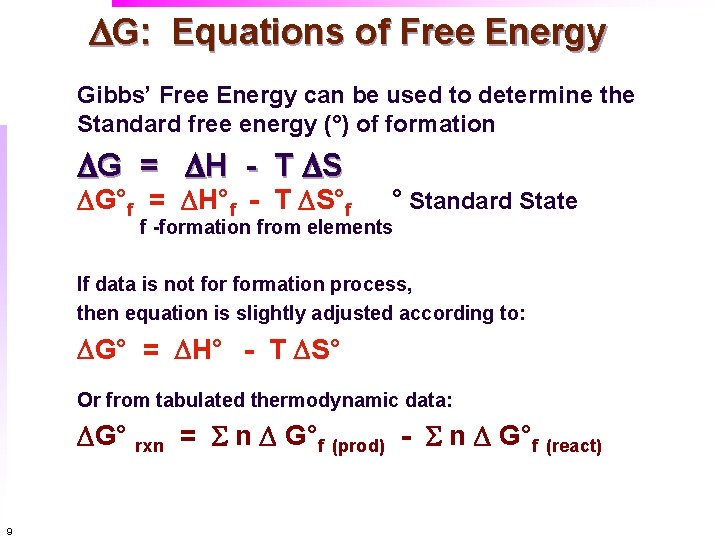
Gibbs Free Energy and Spontaneity and the meaning

Gibbs Free Energy Formula In Electrochemistry Mrschimomot
Gibbs Free Energy Formula In Electrochemistry Mrschimomot
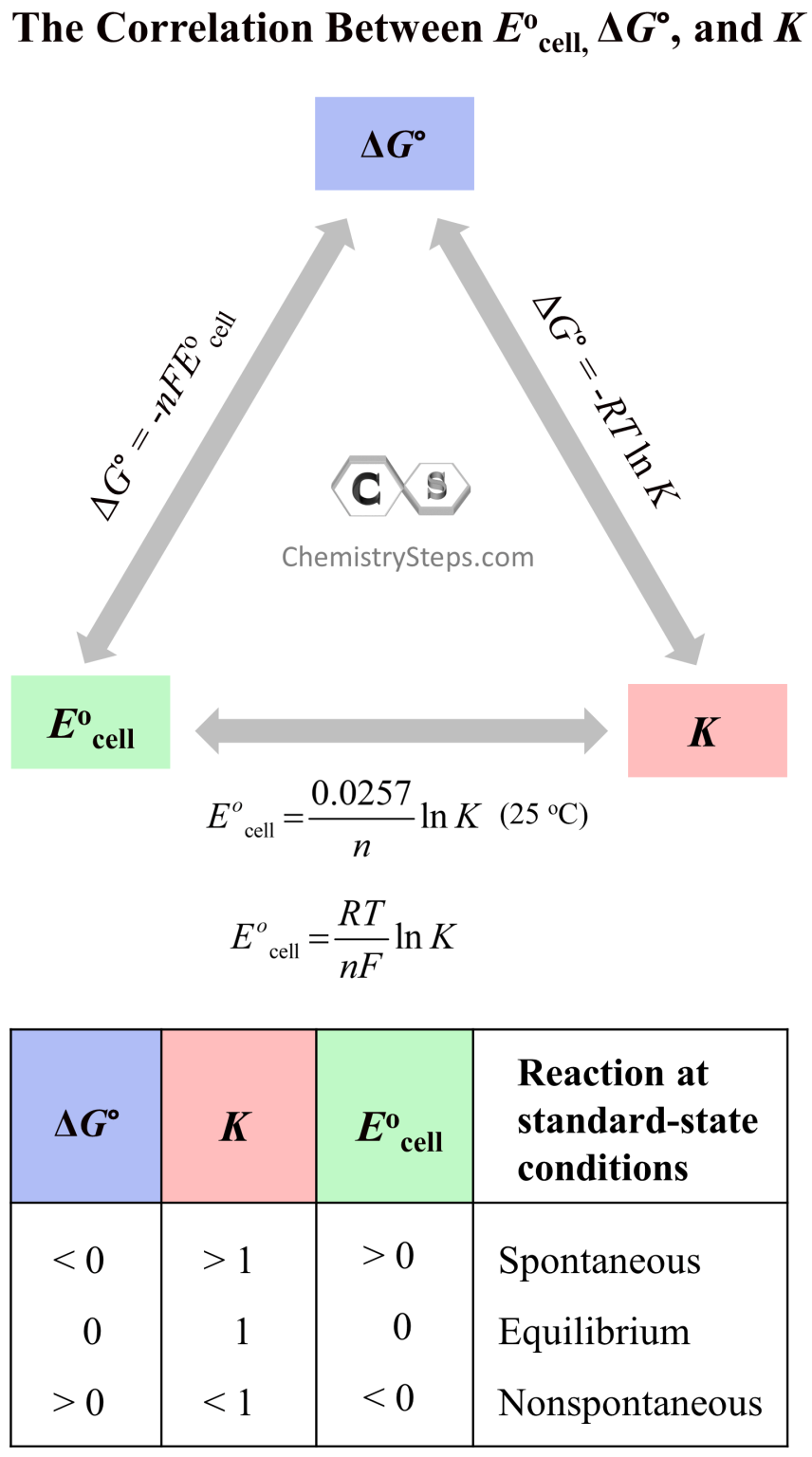
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps

Gibbs Free energy and Cell potentials, Equilbrium constant ll Electrochemistry ll Part 18 ll
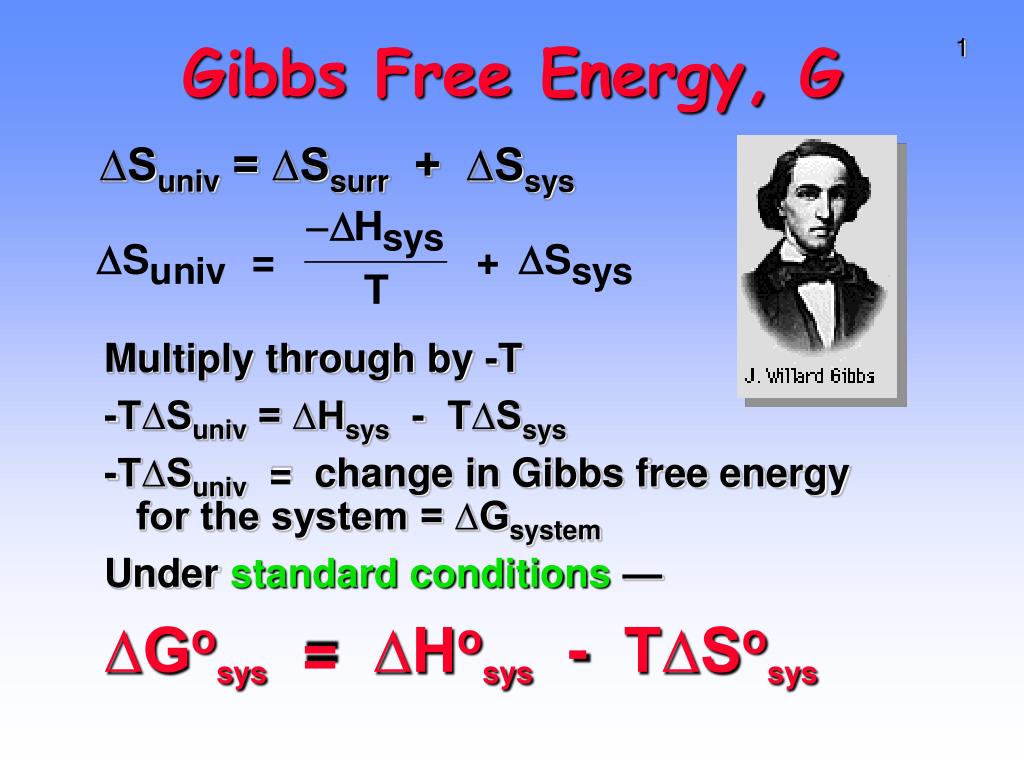
PPT Gibbs Free Energy, G PowerPoint Presentation, free download ID667978
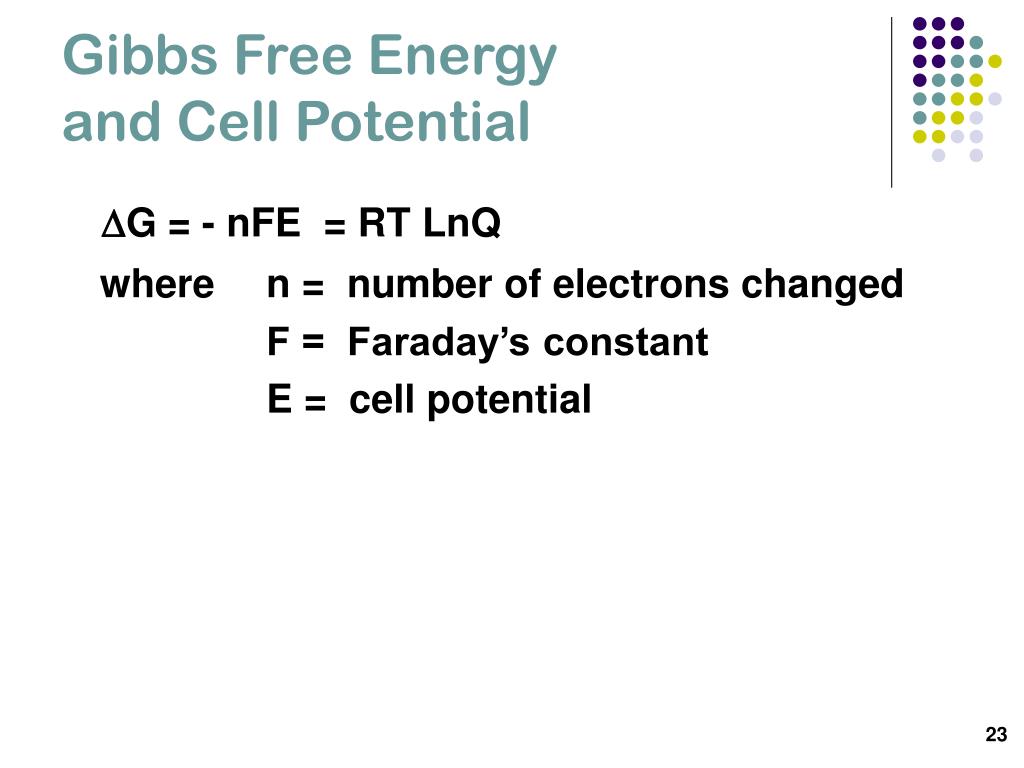
PPT Electrochemistry PowerPoint Presentation, free download ID3975392
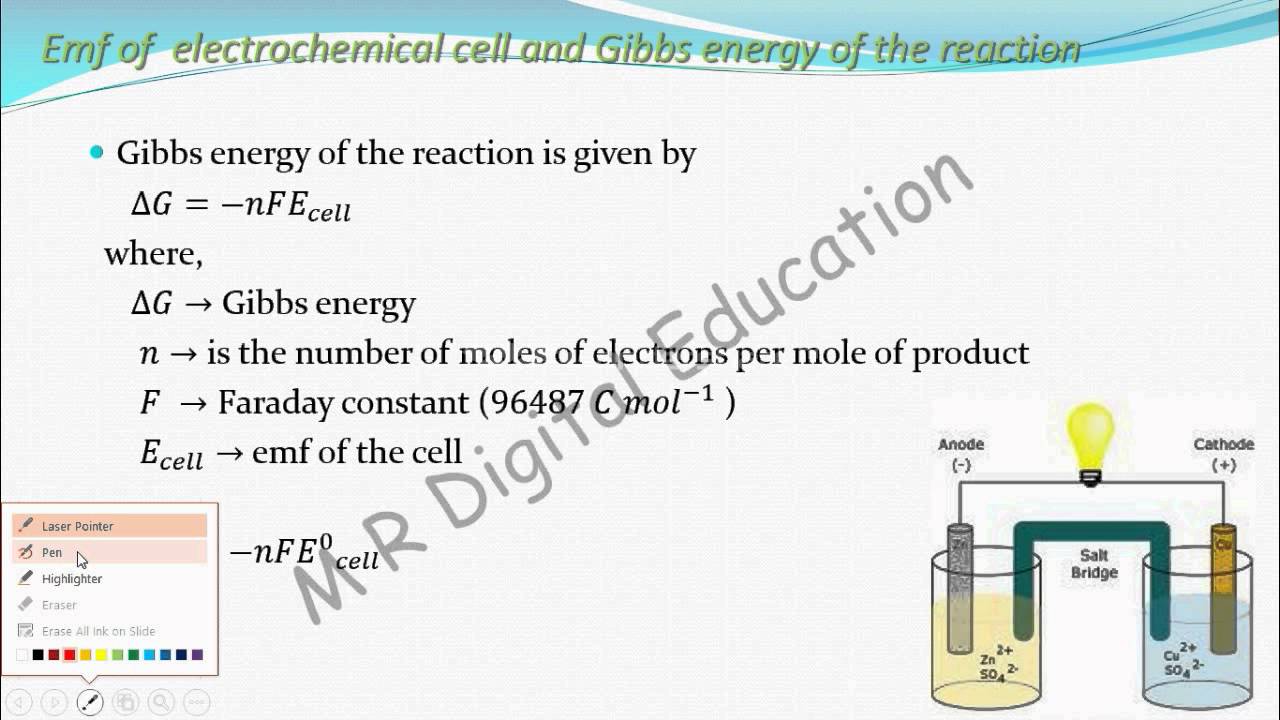
Gibbs free energy (Electrochemistry part 35 for CBSE class 12 JEE IIT) YouTube

Worked examples of Gibbs Free Energy and Electrochemistry YouTube

Gibbs Free Energy Formula In Electrochemistry Mrschimomot

PPT Gibbs Free Energy PowerPoint Presentation, free download ID2198185
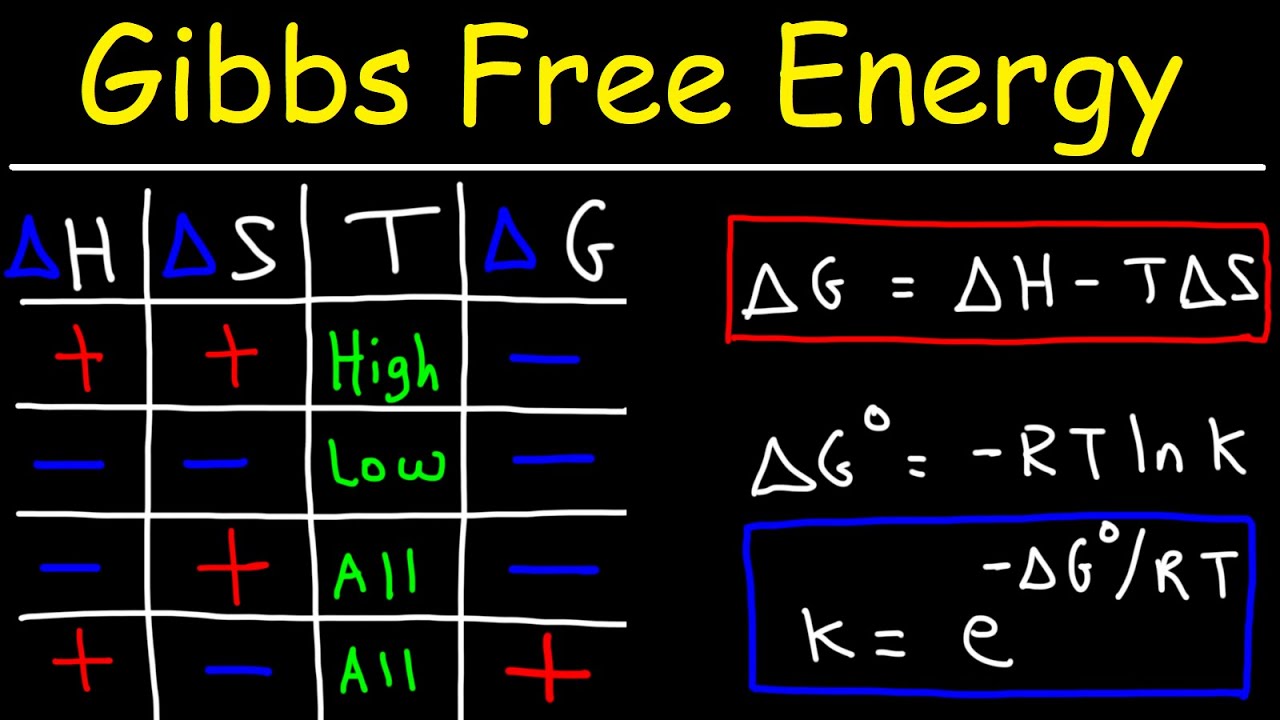
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube

PPT Electrochemistry MAE 212 PowerPoint Presentation ID1588026

Gibbs Free Energy Formula In Electrochemistry Mrschimomot
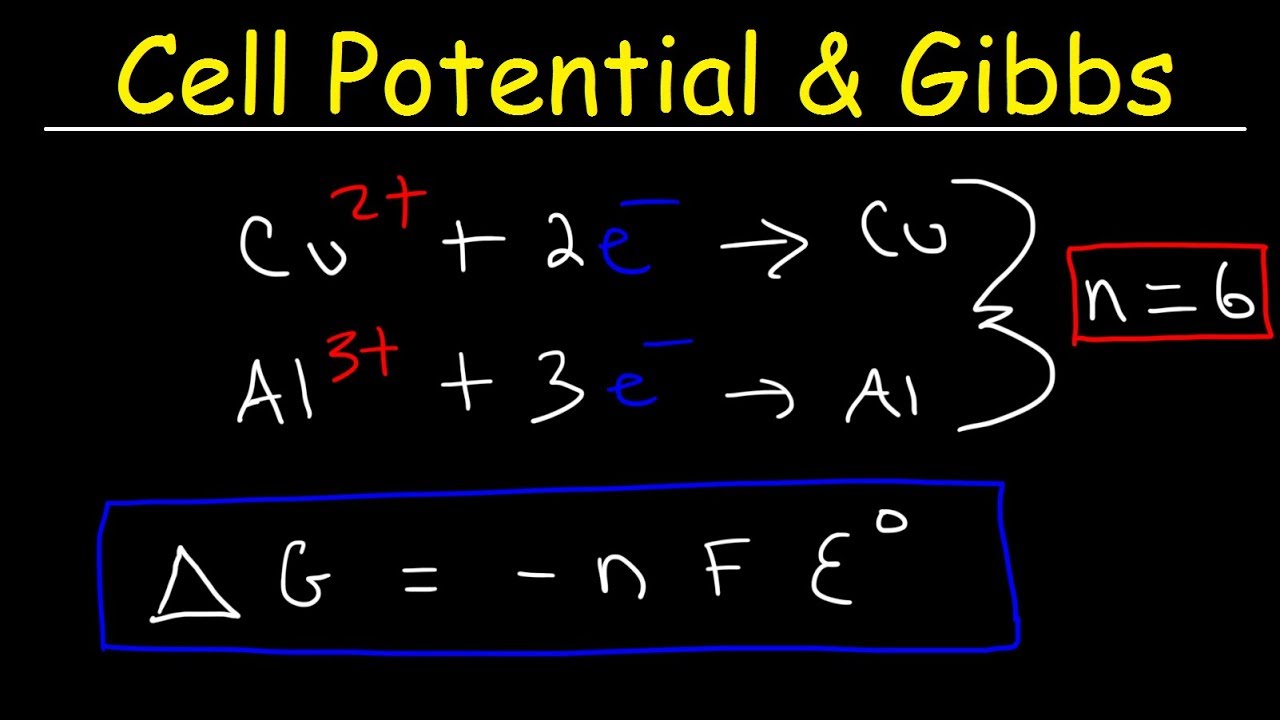
Cell Potential & Gibbs Free Energy, Standard Reduction Potentials, Electrochemistry Problems

A) The Gibbs free energy change for electrochemical synthesis of... Download Scientific Diagram

Electrochemistry 11 Gibb’s Free Energy and Cell Potential Calculation based on it YouTube
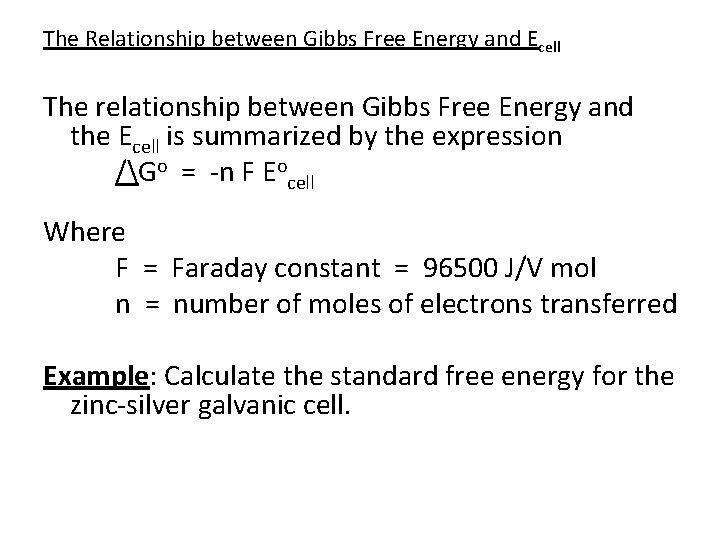
Gibbs Free Energy Formula In Electrochemistry Mrschimomot
Gibbs free energy and spontaneity. When a process occurs at constant temperature T and pressure P , we can rearrange the second law of thermodynamics and define a new quantity known as Gibbs free energy: Gibbs free energy = G = H − TS. where H is enthalpy, T is temperature (in kelvin, K ), and S is the entropy.. Hence, this study delves into the coverage-sensitive mechanism of electrochemical NO reduction on cost-effective perovskite catalysts, using SrTiO3 as an example, through density functional theory calculations.. Gibbs free energy results indicate that the selectivity is significantly influenced by NO coverage. NH3 is likely to be generated.