Question. In a coffee cup calorimeter, 1.60 g of \mathrm { NH } _ { 4 } \mathrm { NO } _ { 3 } NH4NO3 is mixed with 75 .0 g of water at an initial temperature of 25.00^\circ C. 25.00∘C. After dissolution of the salt, the final temperature of the calorimeter content s is 23.34^\circ C. 23.34∘C.. In a coffee cup calorimeter, 1.60 g of NH4NO3 is mixed with 75.0 g. water at an initial temperature of 23.50. Here's the best way to solve it.
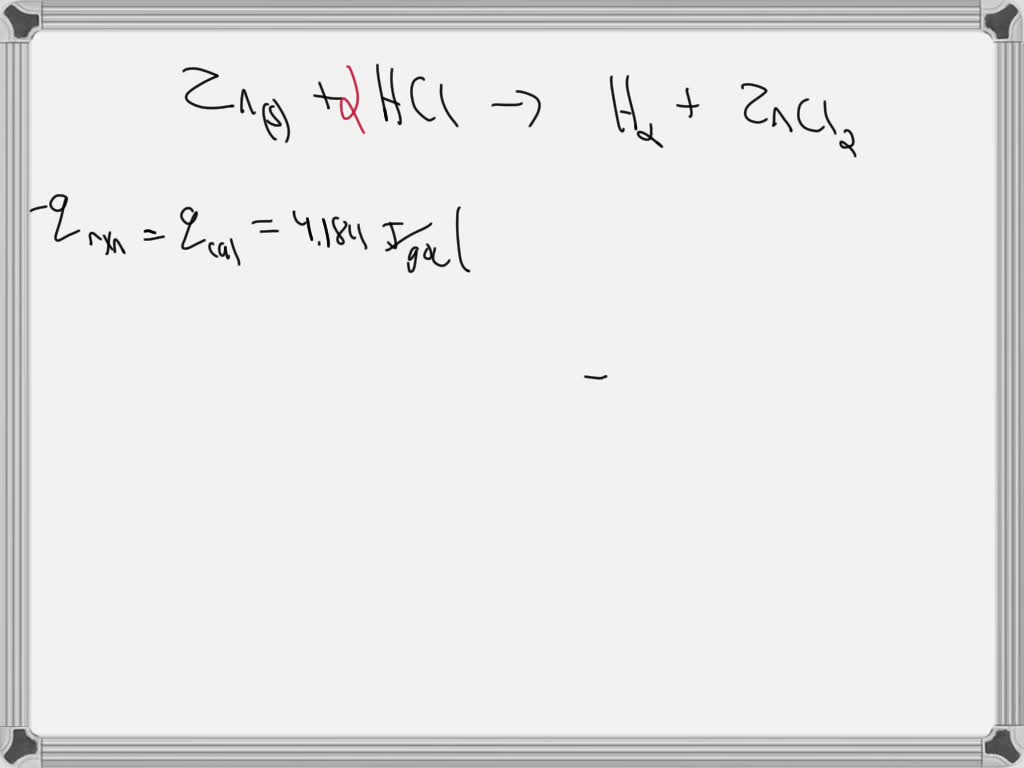
SOLVED A coffeecup calorimeter contains 100.0 mL of 0.300 M HCl at 20.3 °C. When 1.82 g Zn(s
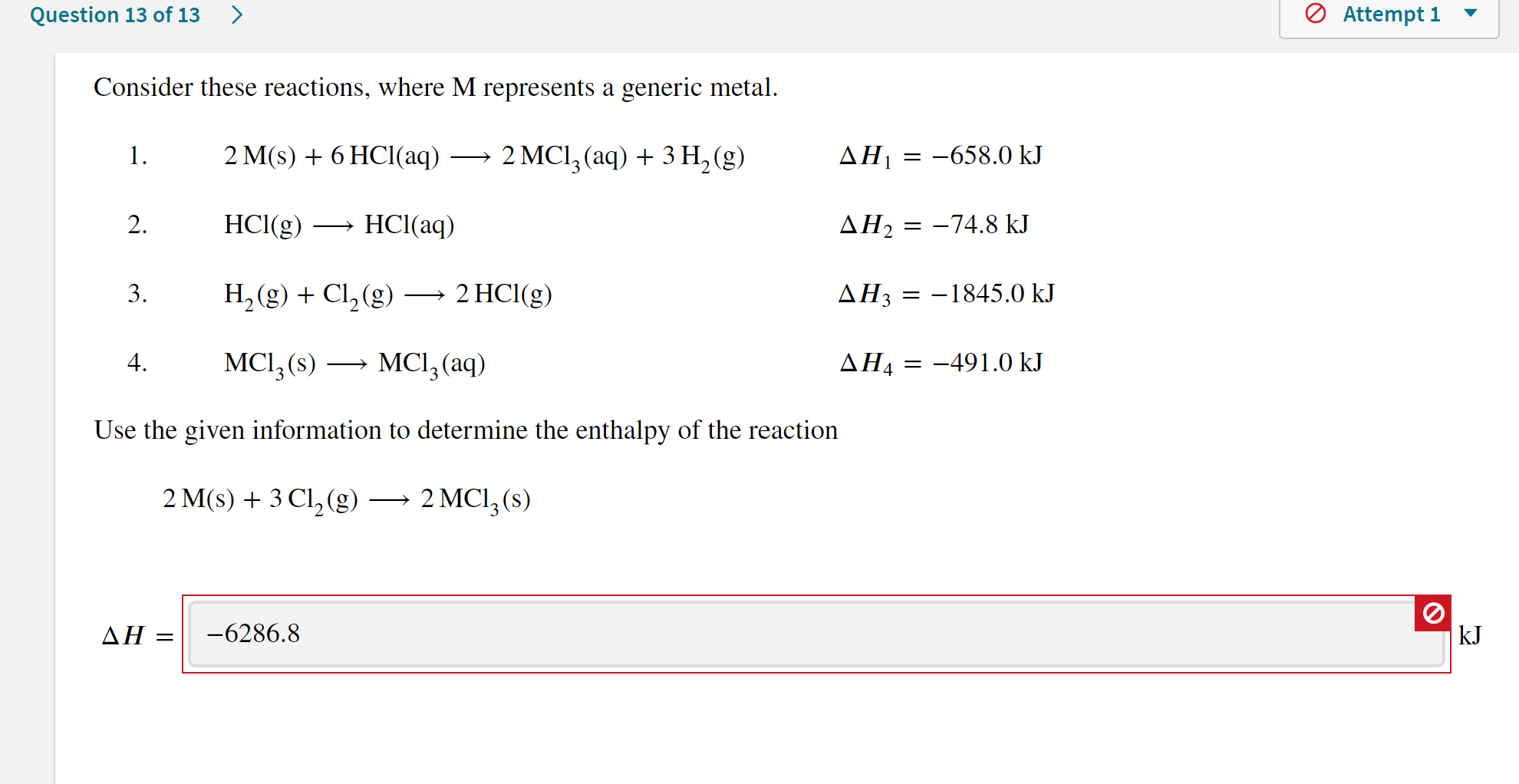
Solved Question 7 of 7 A coffee cup calorimeter with a heat
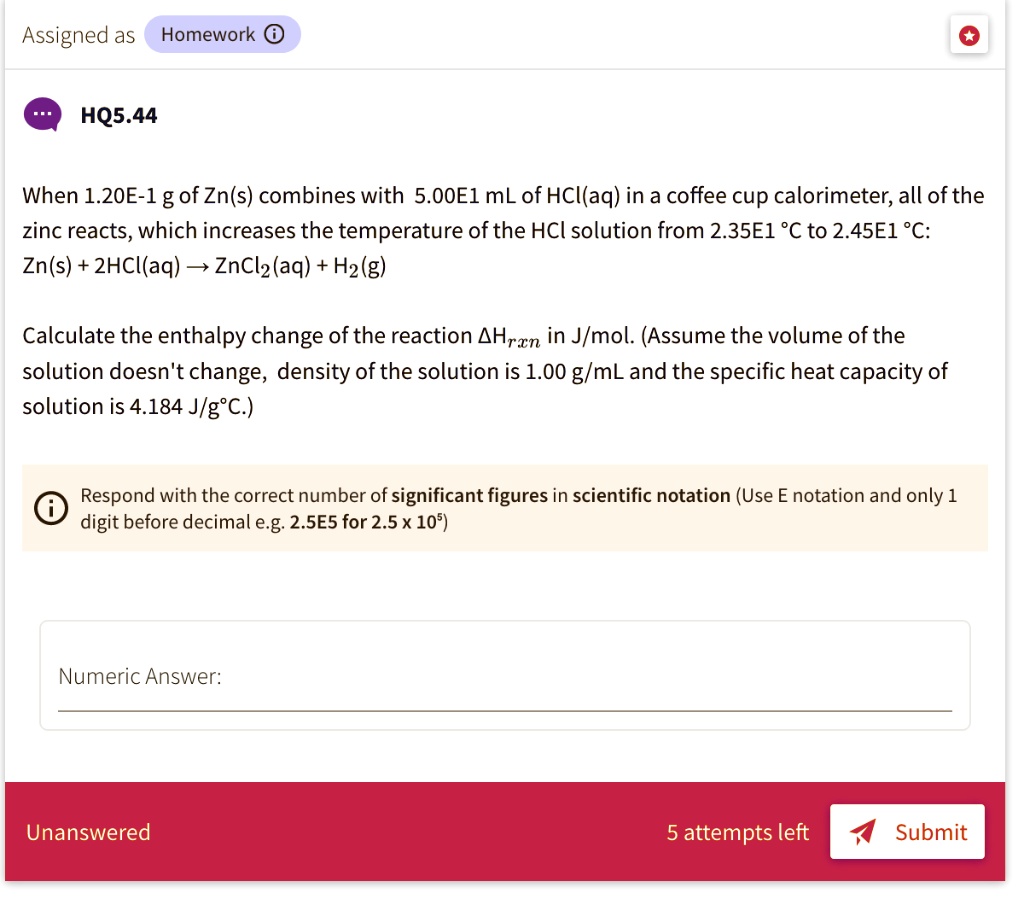
SOLVED Assigned as Homework 0 HQ5.44 When 1.20E1 g of Zn(s) combines with 5.00El mL of HCIaq

Pin on All About Science

In a coffeecup calorimeter, 0.00500 mol of mg is reacted with enough hno3 to produce 100.0 ml

SOLVEDA 14.1mL sample of 0.996 M NaOH is mixed with 32.3 mL of 0.905 M HCl in a coffeecup
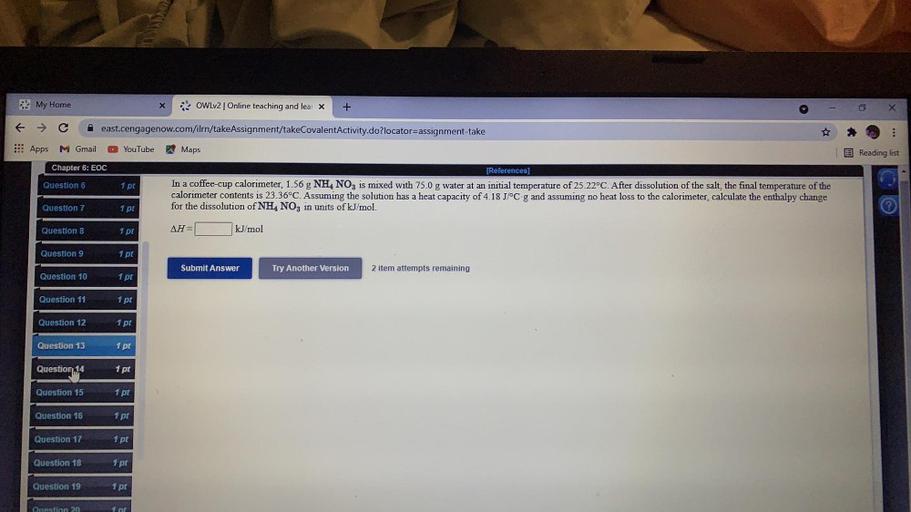
My Home x OWLv2 Online teaching and lear... Chemistry
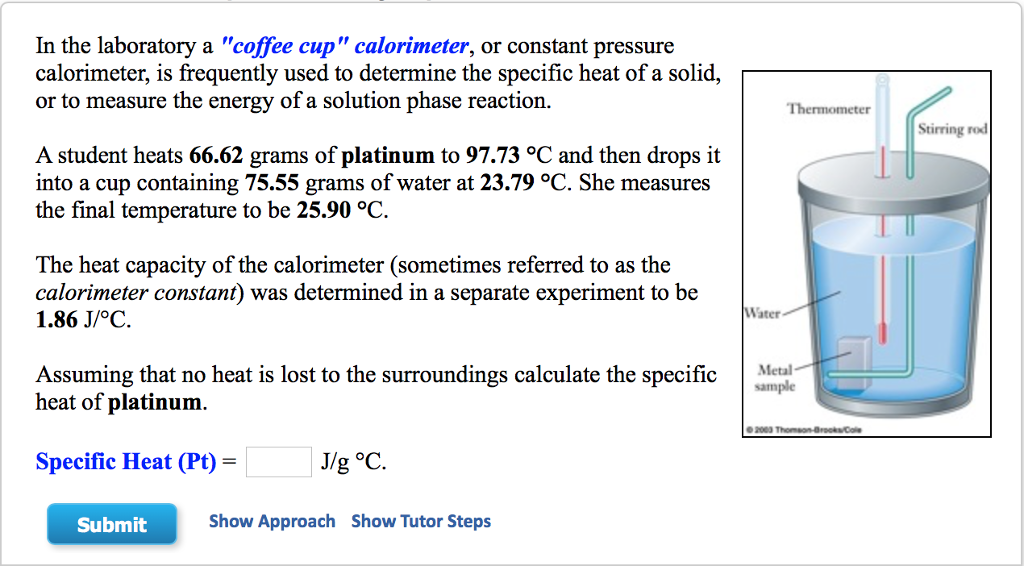
Solved In the laboratory a "coffee cup" calorimeter, or
Chapter 5 Presentation

Solved In a coffeecup calorimeter, 1.80 g of NH_4NO_3 is
Calorimetry · Chemistry
SOLVED In a coffee cup calorimeter, 1.60 g NH4NO3 was mixed with 75.0 grams of water at an

in this lab we will build an instrument called a coffee cup calorimeter identify the list with
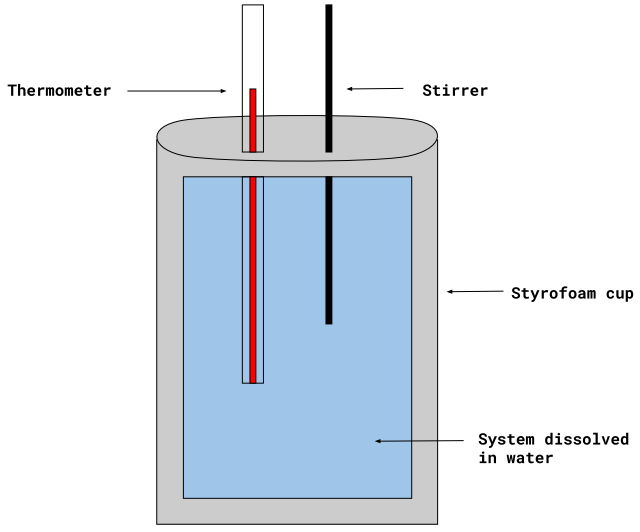
Understanding Coffee Cup Calorimetry
[Solved] In a coffeecup calorimeter, 3.868 g of NH4NO3 (MW = 80.04) is... Course Hero
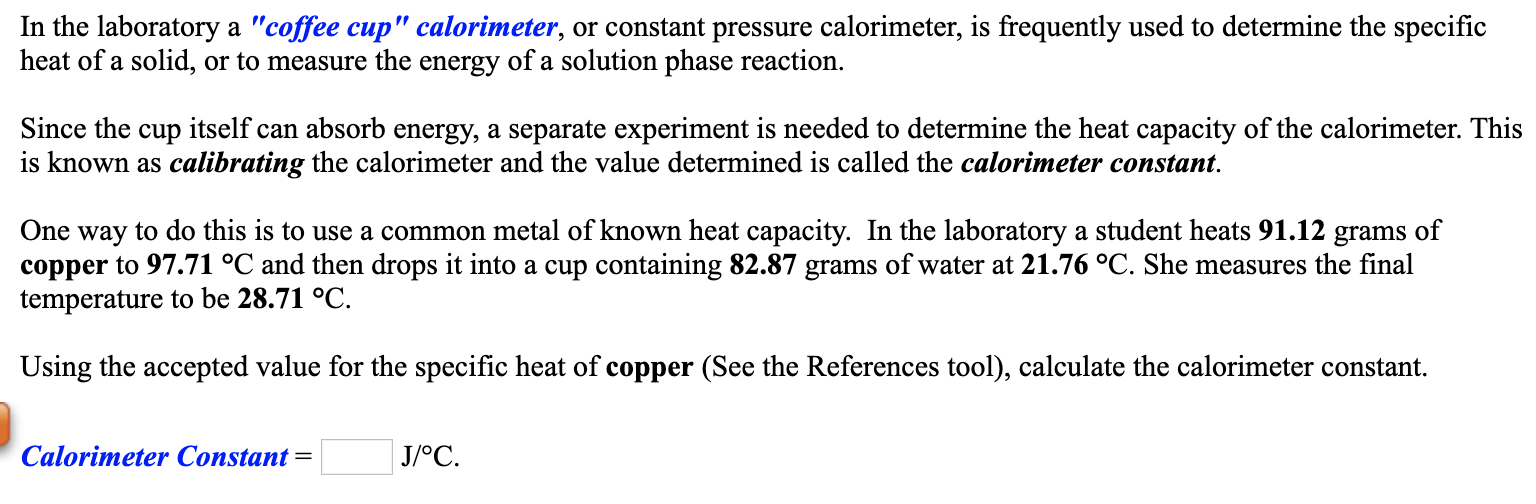
Solved In the laboratory a "coffee cup" calorimeter, or

Enthalpy changes in solution
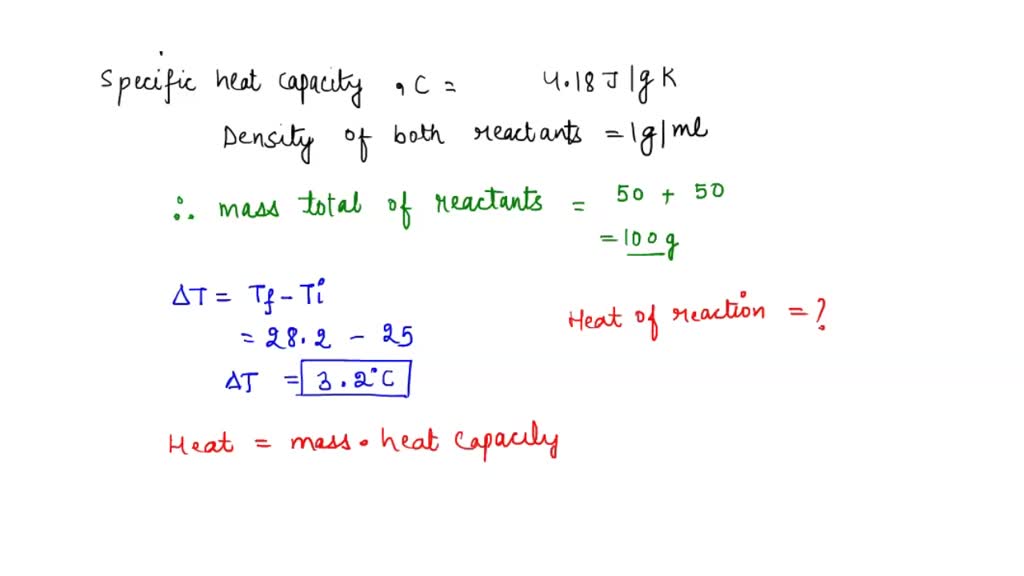
SOLVED A 50.0mL sample of 0.250 M HCl is added to a 50.0mL sampleof 0.250 M NaOH in a

Coffee Cup Calorimeter Diagram

SOLVED In a coffeecup calorimeter, 1.70 g of NH4NO3 is mixed with 72.0 g of water at an
In a coffee cup calorimeter, 1.60 g NH4NO3 was mixed with 75.0 grams of water at an initial temperature of 25.00* Celsius. After the dissolution of the salt, the final temperature of the calorimeter contents was 23.34 * Celcius. Assuming the solution has a heat capacity of 4.18 J/g*C, and assuming no heat loss to the calorimeter, calculate the.. In a coffee-cup calorimeter, 1.91 g of NH4NO3 is mixed with 75.0 g of water at an initial temperature of 25.00 degree Celsius. After the solution of the salt, the final temperature was 20.94 degree Ce; In a coffee cup calorimeter, 1.60 g of NH_4NO_3 is mixed with 75.0 g water at an initial temperature of 23.50 degrees C.