Sodium sulfate. Molecular Formula NaOS. Average mass 142.042 Da. Monoisotopic mass 141.931274 Da. ChemSpider ID 22844. - Charge.. Sodium sulfate. Formula: Na 2 O 4 S; Molecular weight: 142.042;. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Species with the same structure: sodium sulphate; Information on this page: Notes; Other data available: IR Spectrum;
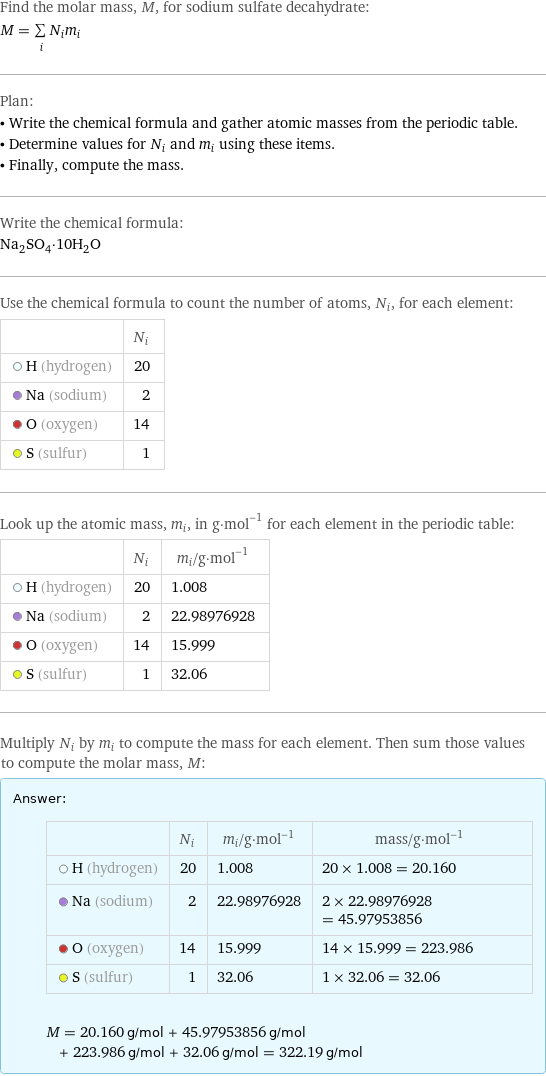
molar mass of sodium sulfate decahydrate

what is the mass of 10 moles of sodium sulphate (Na2so3 Brainly.in

SOLVED For the following chemical reaction, what mass of sodium sulfate (in grams) will be

Molar Mass / Molecular Weight of Al2(SO4)3 (Aluminum Sulfate) YouTube

sodium Facts, Uses, & Properties Britannica
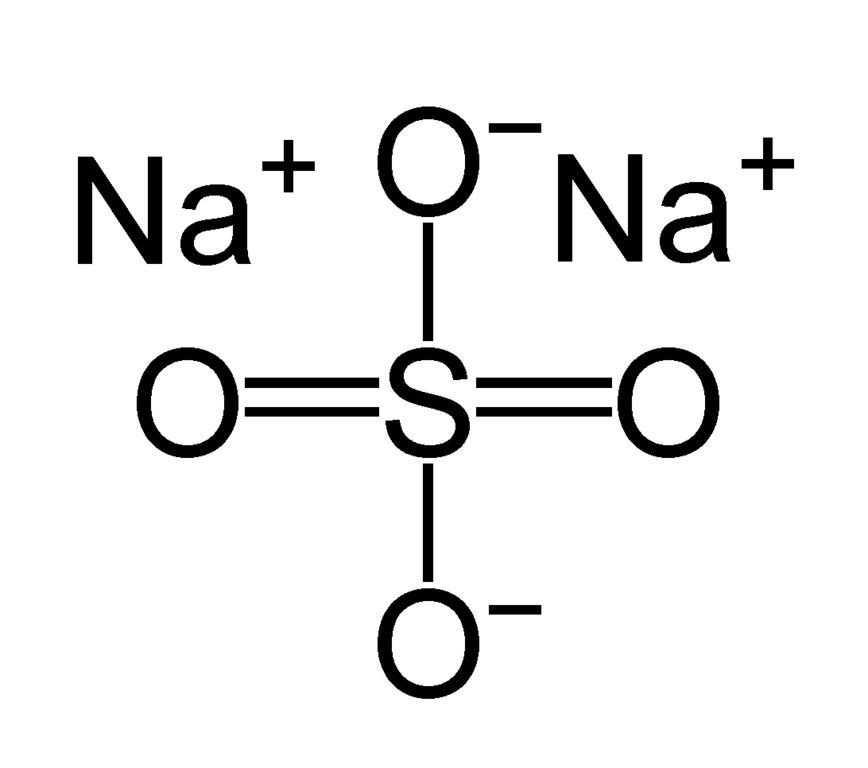
Original file (1,092 × 966 pixels, file size 4 KB, MIME type image/png )

Molar Mass Of Sodium Sulfate slidesharedocs
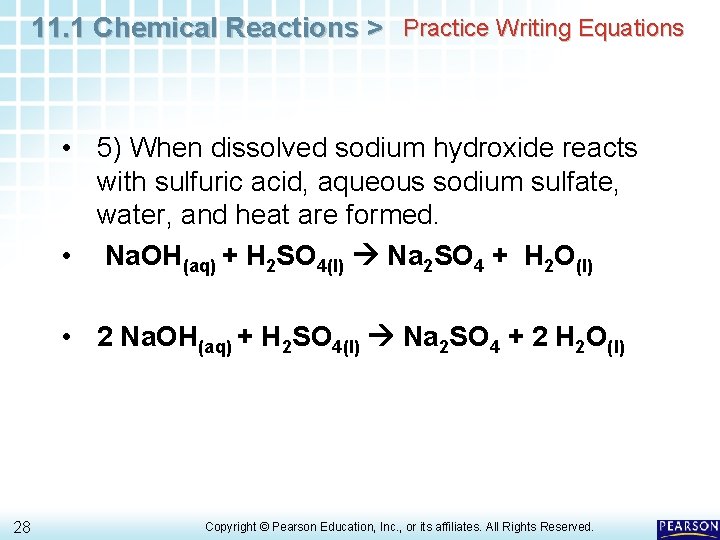
Balanced Chemical Equation For Sodium Sulfate And Water Tessshebaylo
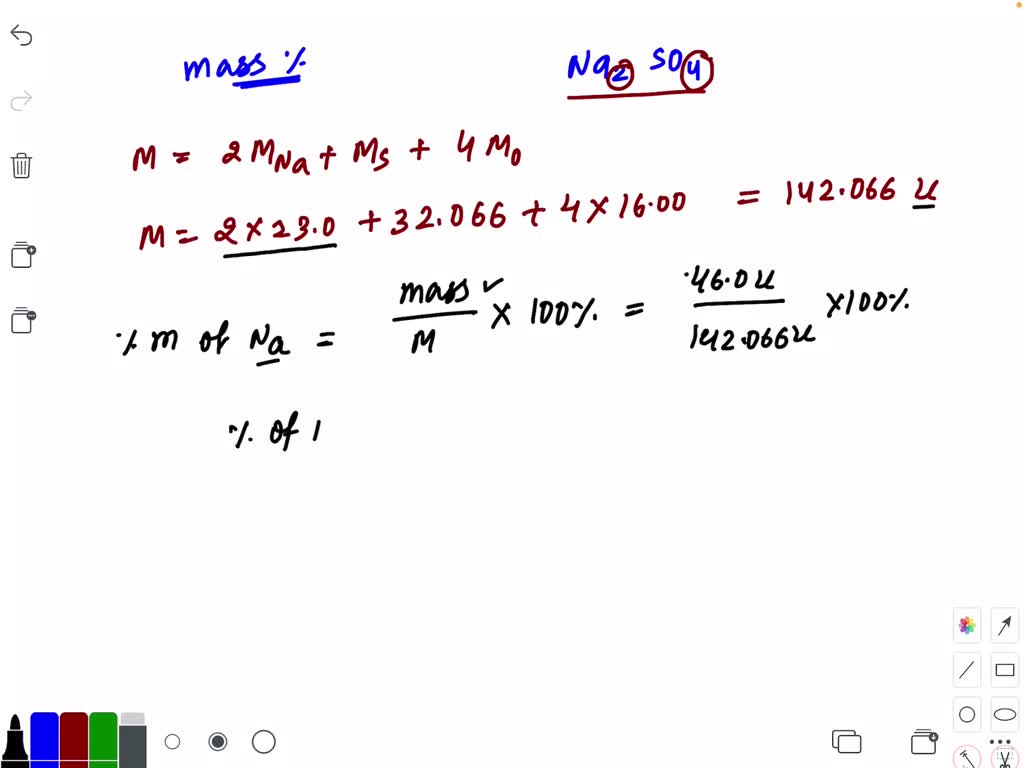
SOLVEDcalculate the molar mass f sodium sulfate

Solved Question 4 1 pts How many grams of sodium sulfate are

Calculate the molar mass for sodium sulfate, A sample of sodium sulfate with mass of represents
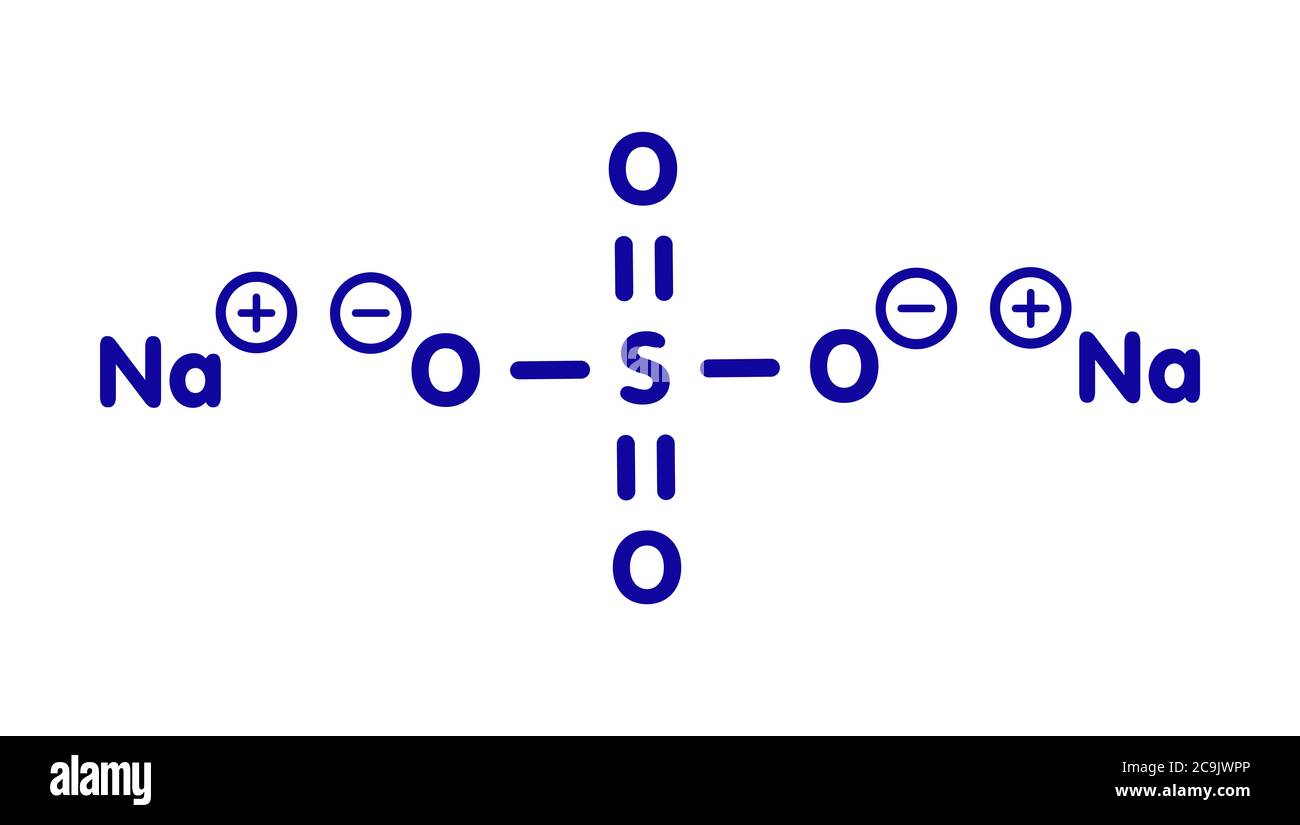
Na2so4 Cut Out Stock Images & Pictures Alamy

Molar Mass / Molecular Weight of Na2SO4 (Sodium sulfate) YouTube
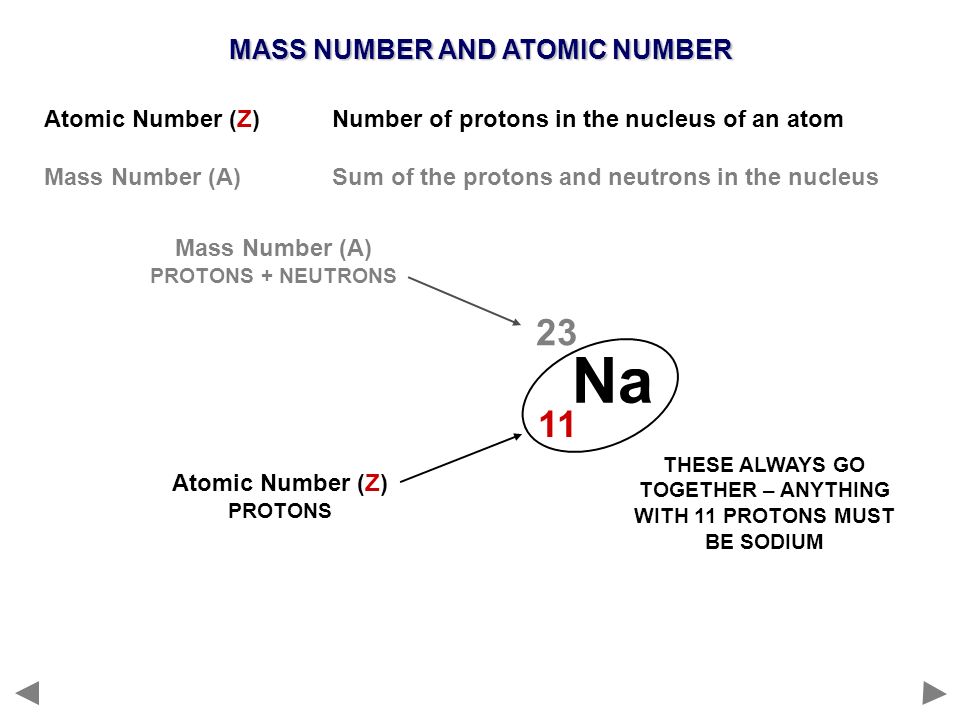
Sodium Mass Number and Atomic Number Dynamic Periodic Table of Elements and Chemistry

Molar Mass Of Sodium Sulfate slidesharedocs
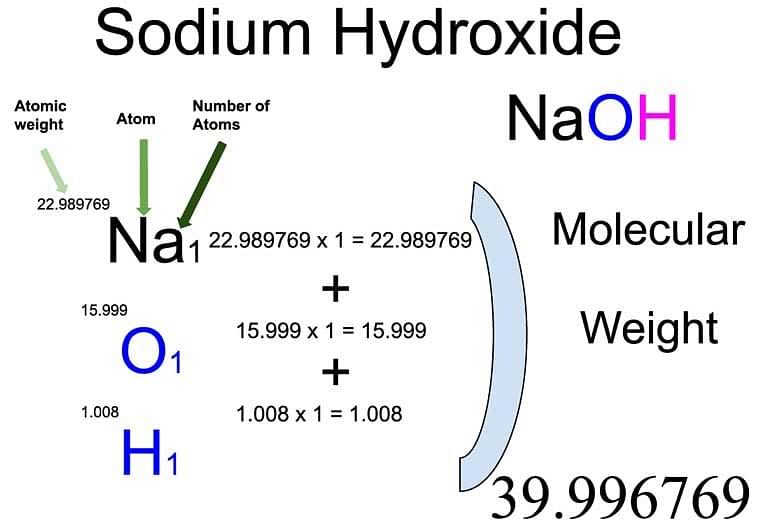
What is the molar mass of Sodium hydroxide?

Sodium Sulfate Molecular Structure
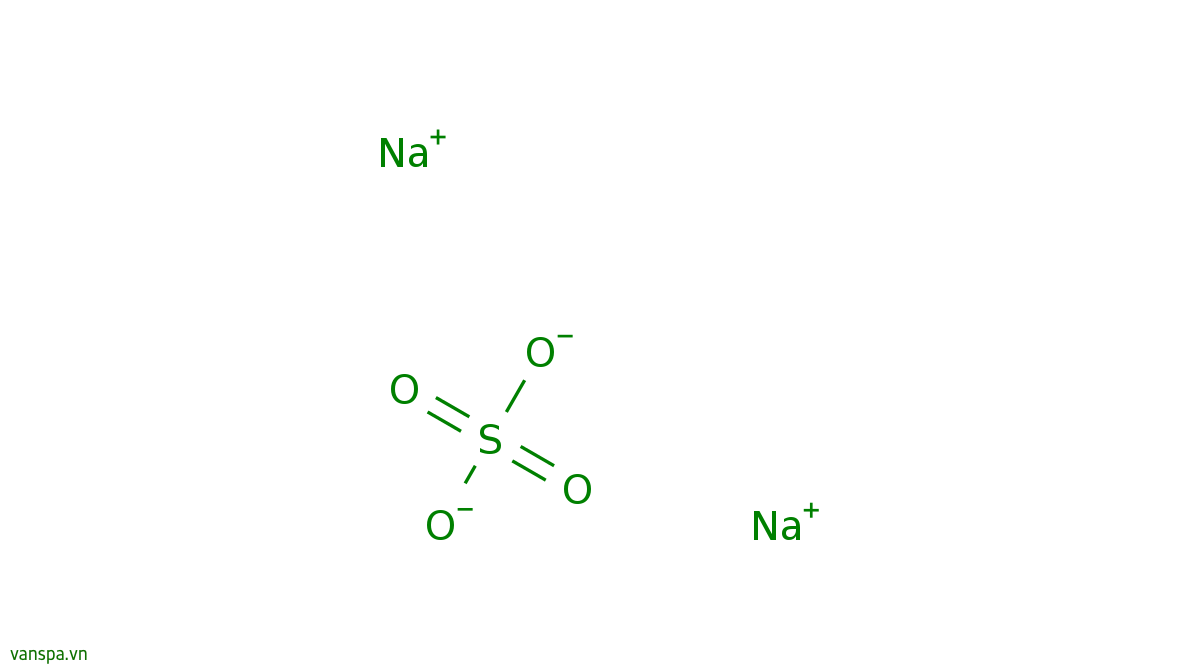
Sodium Sulfate

Molecular Formula Sodium Thiosulfate Chemical Structure Stock Vector (Royalty Free) 2017793030

Sodium sulfate, 99+, ACS reagent, granular, anhydrous, ACROS Organics™ Compuestos
You can see that in Na2SO4, there are 2 Sodium atoms, 1 Sulfur atom and 4 Oxygen atoms. So, Molar mass of Na2SO4 = Molar mass of 2 Sodium (Na) atoms + Molar mass of 1 Sulfur (S) atom + Molar mass of 4 Oxygen (O) atoms. Hence the Molar mass of Na2SO4 is 142.036 g/mol. I hope you have understood the short and simple calculation for finding the.. Finally, add together the total mass of each element to get the molar mass of Na2SO4: 45.97953856 g/mol + 32.065 g/mol + 63.9976 g/mol = 142.04213856 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in Na2SO4, divide each total from step 3 by the total molar mass found in step 4: