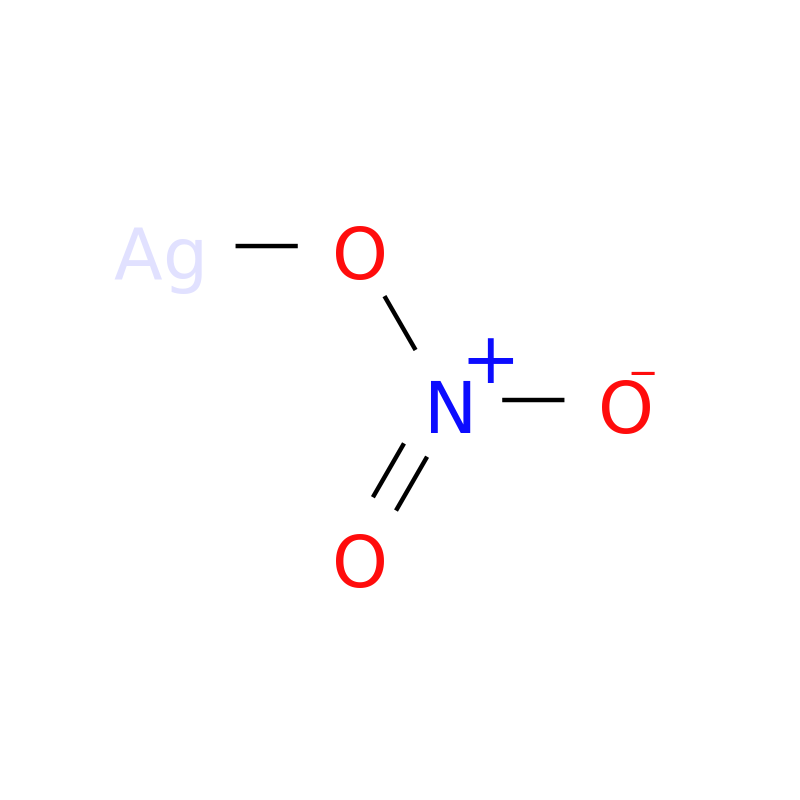
The molar mass and molecular weight of NO3Ag (Silver Nitrate) is 169.873. ChemicalAid. ⚛️ Elements. Periodic Table; Periodic Trends; Element Charts; Future Elements; 🛠️. The first step to finding the molar mass of Silver Nitrate is to count the number of each atom present in a single molecule using the chemical formula, NO3Ag.. Finally, add together the total mass of each element to get the molar mass of AgNO3: 107.8682 g/mol + 14.0067 g/mol + 47.9982 g/mol = 169.8731 g/mol. 5. Find Mole Fraction. To find the mole fraction and percentage of each element in AgNO3, divide each total from step 3 by the total molar mass found in step 4:

Silver Nitrate HCS Scientific & Chemical Pte Ltd

Using Criss cross method find the molecular formola of Silver nitrate Brainly.in

Silver Nitrate Laboratory Chemical at Rs 65000/kg Silver Nitrate in Chennai ID 12294698788

The value of observed and calculated molecular weight of silver nitrate are 90, 2.64 and 170
Silver nitrate brand name list from

The value of the observed and calculated molecular weight of silver nitrate is 92.64 and 170
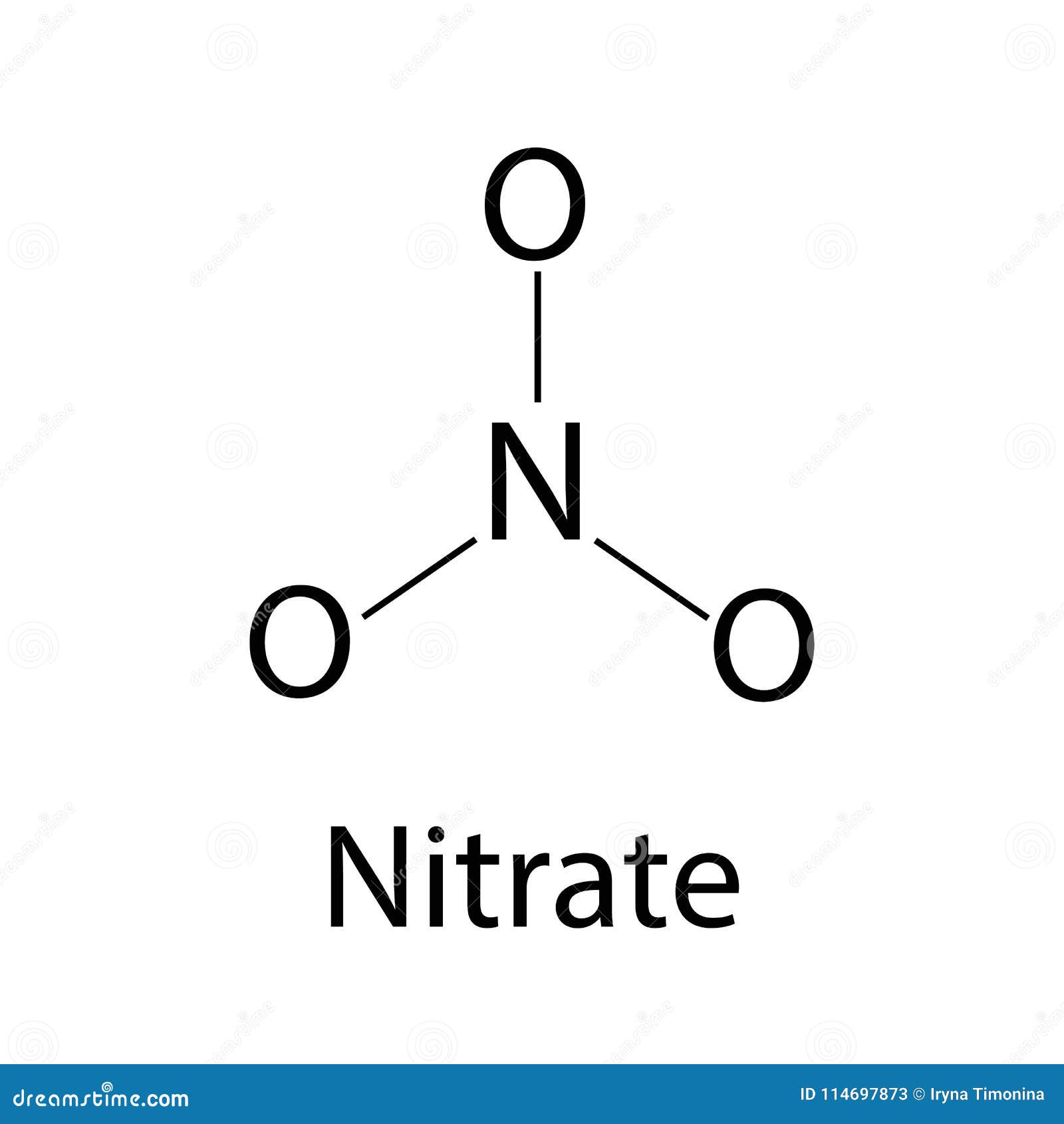
The Chemical Formula Of Nitrate. Infographics. Vector Illustration On Isolated Background

Silver Nitrate Stock Image C028/0901 Science Photo Library

Silver Nitrate, 5 Solution, 100ml Chemsavers, Inc.

What is the molar mass of silver nitrate? YouTube
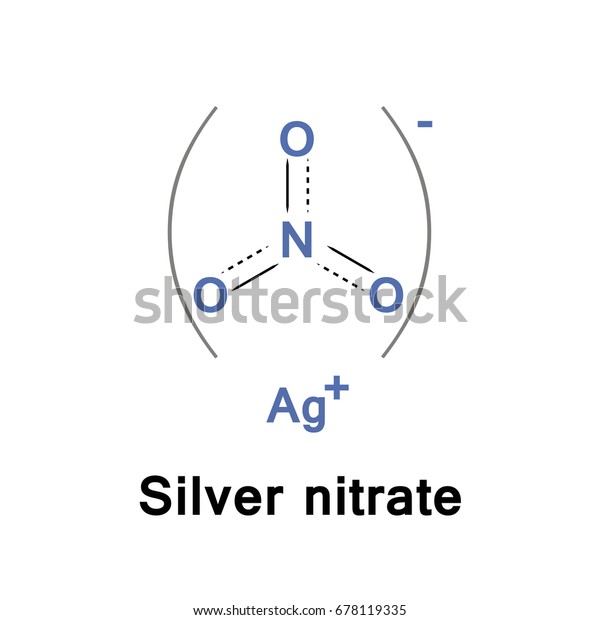
Silver Nitrate Compound Chemical Formula Stock Vector (Royalty Free) 678119335

Sodium Iodide & Glyceryl Monostearate Exporter from Mumbai

Silver Nitrate Chemical Formula SexiezPix Web Porn

In the reaction of silver nitrate with sodium chlorid… SolvedLib

Silver Nitrate Symbol Periodic Table Awesome Home

Calculate molecular weight of Silver nitrateMolaeular mass Silver nitrateMolar mass AgNO3

The values of observed and calculated molecular weight of silver nitrate are 92.64 and 172

Silver Nitrate 99 powder lab grade 50 gr Etsy

The Science Company Silver Nitrate Crystals, Reagent, 25g Albochemicals Lab and Science Supplies

PPT Preparation of silver nitrate and its uses PowerPoint Presentation ID3003485
Physical Properties: As a solid, it forms transparent rhombohedral crystals. It has a melting point of 212 degrees Celsius and a boiling point of 440 degrees Celsius. Its density is 4.35 g/cm 3 at 25 degrees Celsius. Chemical Properties: Silver nitrate is highly reactive. It can react with many salts to precipitate silver salts.. The molecular weight of Silver nitrate [AgNO 3] is 169.87314.. Silver nitrate [AgNO 3] is an inorganic compound of three elements: Silver, Nitrogen, and Oxygen.The molecular weight of Silver nitrate is 169.87314 which can be calculated by adding up the total weight (atomic weight multiplied by their number) of Silver, Nitrogen, and oxygen.